How Many Valence Electrons Are in Silver
Significant concentrations of boron occur on the Earth in compounds known as the borate minerals. 557 3 3 gold badges 8 8 silver badges 10 10 bronze badges endgroup 1 begingroup You might find this answer of mine useful.

How To Find The Valence Electrons For Silver Ag Youtube
The valence band has the highest occupied energy.
. The second orbit is now full. The p-orbital can have a maximum of six electrons. Therefore the next two electrons enter the 2s orbital.
Theres an important distinction between the number of electrons possible in a shell and the number of valence electrons possible for a period of elements. The p-orbital can have a maximum of six electrons. The s-orbital can have a maximum of two electrons.
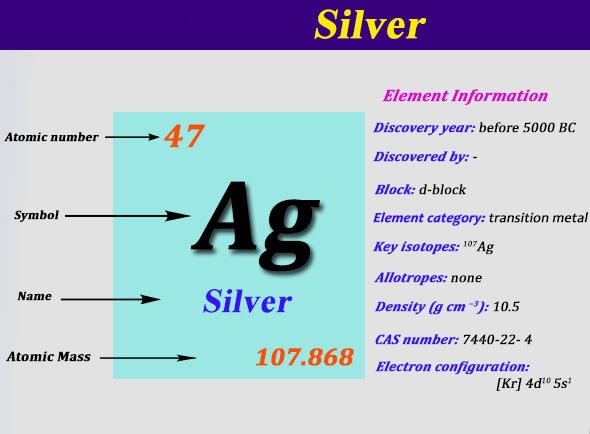
The electrons in the outermost shell are known as valence electrons. Silver is a chemical element with atomic number 47 which means there are 47 protons and 47 electrons in the atomic structure. There are many examples of solids that have a single bonding type while other.
Hydrogen-bonded solids such as ice make up another category that is important in a few crystals. At this point no current flowsthat is no significant movement of electrons through the wire occurs because the circuit is open. The first two electrons of manganese enter the 1s orbital.
CSUS Chemistry 1A Nomenclature Worksheet Dr. The second orbit is now full. The silver is undergoing reduction.
So the remaining electrons will enter the third orbit. Seven valence electrons so elements from this group typically exhibit a -1 oxidation state. Mack Page 3 of 9 S S2 sulfide ion IA H H hydride ion Polyatomic Ions Polyatomic ions are ions that are composed of two or more atoms that are linked by covalent bonds but that still have a net deficiency or surplus of electrons resulting in an overall charge on the group.
The properties of a solid can usually be predicted from the valence and bonding preferences of its constituent atoms. These valence electrons contain a series of energy levels and form an energy band known as valence band. Silver is an exception and only makes an oxidation.
Ionic covalent metallic and molecular. Unlike other groups noble gasses are unreactive and have very low electronegativity or electron affinity. Valence electrons arranged as ns2np6 where n corresponds to the main energy level.
The noble gasses have complete valence electron shells so they act differently. The elements in the boron group are characterized by having three valence electrons. The number of electrons in each elements electron shells particularly the outermost valence shell is the primary factor in determining its chemical bonding behavior.
As the temperature increases this electron collision process becomes faster which results in increased resistance with the rise in the temperature of the conductor. Possible oxidation states are 1. Electron Configurations Orbital Notation and Quantum Numbers 318 Laying the Foundation in Chemistry 5.
The boron group are the chemical elements in group 13 of the periodic table comprising boron B aluminium Al gallium Ga indium In thallium Tl and perhaps also the chemically uncharacterized nihonium Nh. The nucleus is composed of protons and neutrons. There are over 100 different borate minerals but the most common are.
Borax kernite ulexite etc. Four main bonding types are discussed here. Therefore the next two electrons enter the 2s orbital.
The half-cell on the right side of the figure consists of the silver electrode in a 1 M solution of silver nitrate AgNO 3. The chemical symbol for Silver is Ag. Boron is a chemical element with atomic number 5 which means there are 5 protons and 5 electrons in the atomic structure.
Gold Aluminium Silver Copper all these metals allow an electric current to flow through them. So the remaining electrons will enter the third. The electrical resistance of conductors such as silver copper gold aluminum etc depends on electrons collision process within the material.
Electron configuration of Silver is Kr 4d10 5s1. Therefore the silver electrode is the cathode. In the periodic table the elements are listed in order of increasing atomic number Z.
The atom consist of a small but massive nucleus surrounded by a cloud of rapidly moving electrons. The s-orbital can have a maximum of two electrons. So the next six electrons enter the 2p orbital.
So the next six electrons enter the 2p orbital. These elements have also been referred to as the triels. Theres space for 18 texte- in the 3rd shell.
The first two electrons of krypton enter the 1s orbital. Metallic bonding is a type of chemical bonding that arises from the electrostatic attractive force between conduction electrons in the form of an electron cloud of delocalized electrons and positively charged metal ionsIt may be described as the sharing of free electrons among a structure of positively charged ions Metallic bonding accounts for many physical properties. Metal definition any of a class of elementary substances as gold silver or copper all of which are crystalline when solid and many of which are characterized by opacity ductility conductivity and a unique luster when freshly fractured.
The chemical symbol for Boron is B.

2022 Valence Electrons In Silver Ag Facts Color Discovery

How Many Valence Electrons Does Silver Have Archives Dynamic Periodic Table Of Elements And Chemistry

How Many Valence Electrons Does Carbon Have Perfect Atom Electrons Electron Configuration Business Plan Template

Comments
Post a Comment